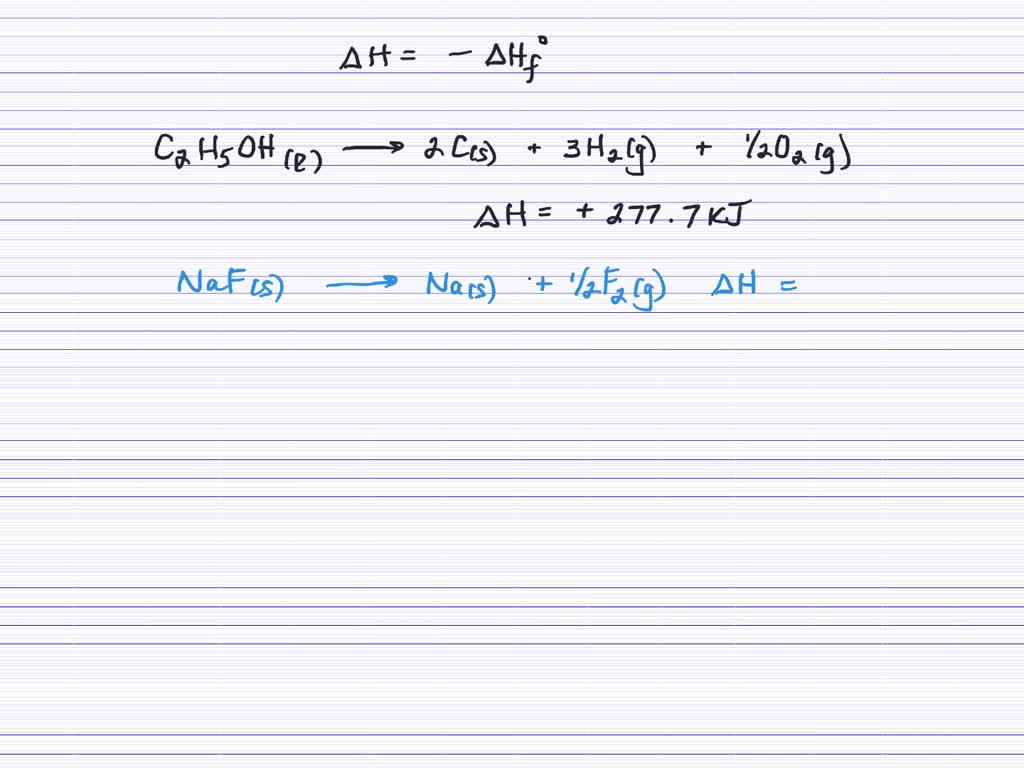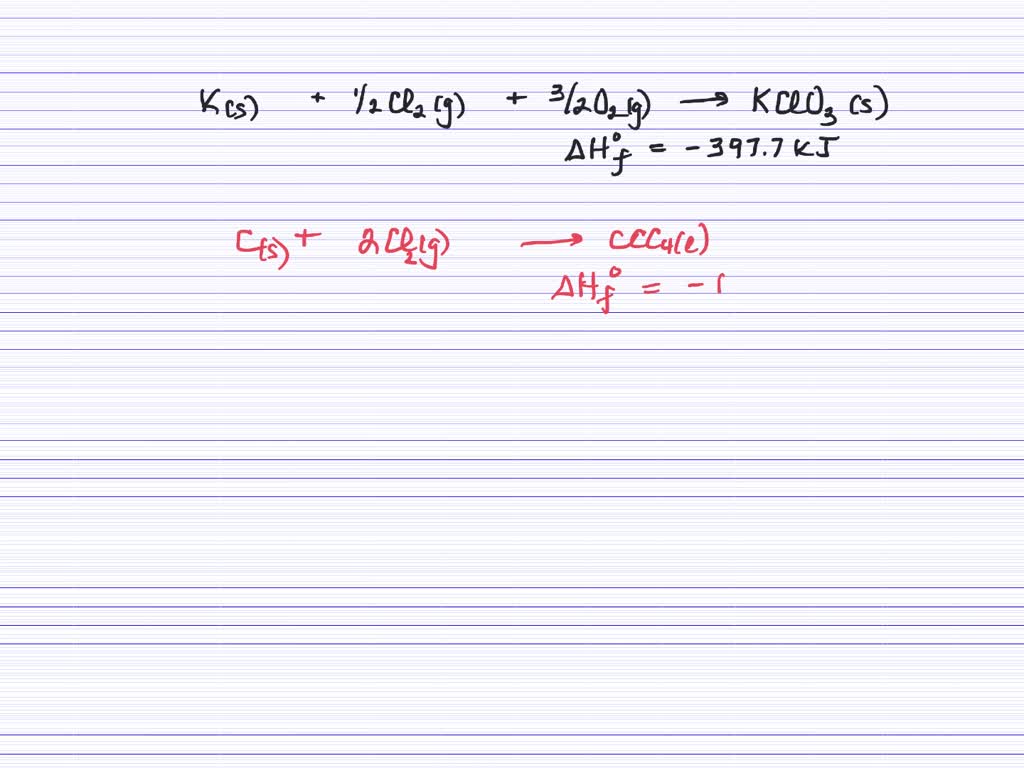Peerless Info About How To Write Thermochemical Equations
The process in the above thermochemical equation can be.
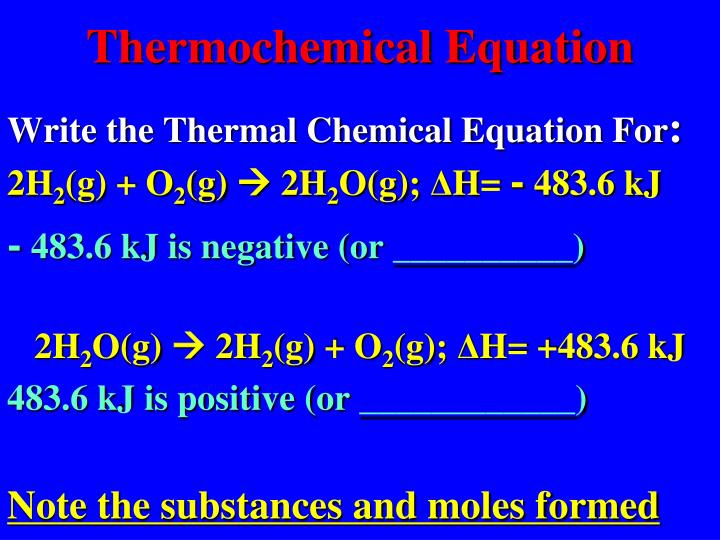
How to write thermochemical equations. Start with a balanced chemical equation: There are a few key things that you need in order to write out a. You already know how to write and balance chemical equations.
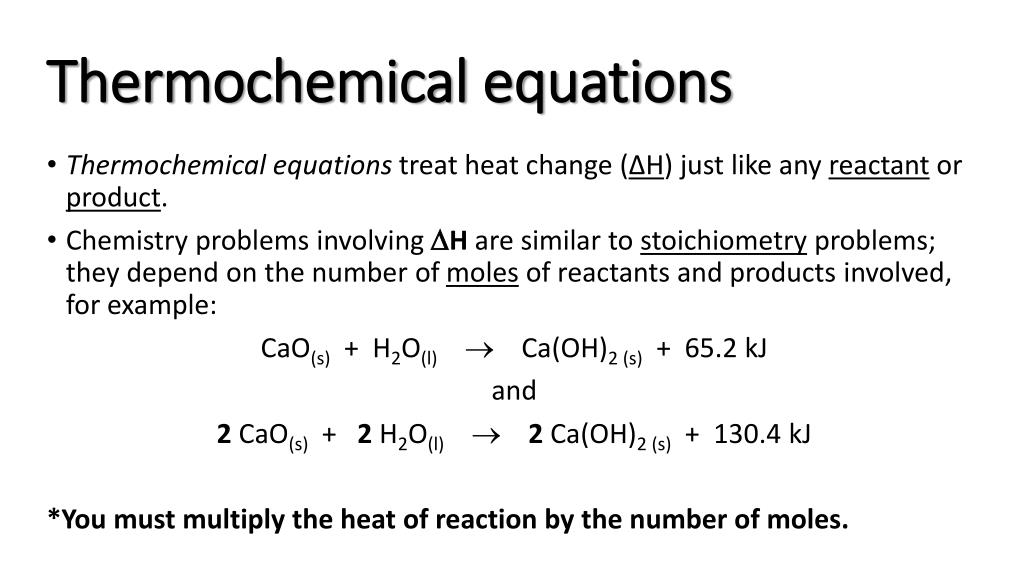
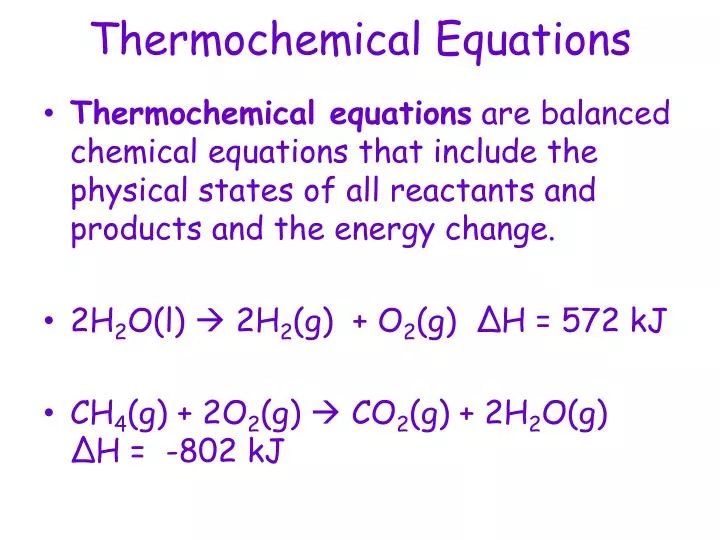
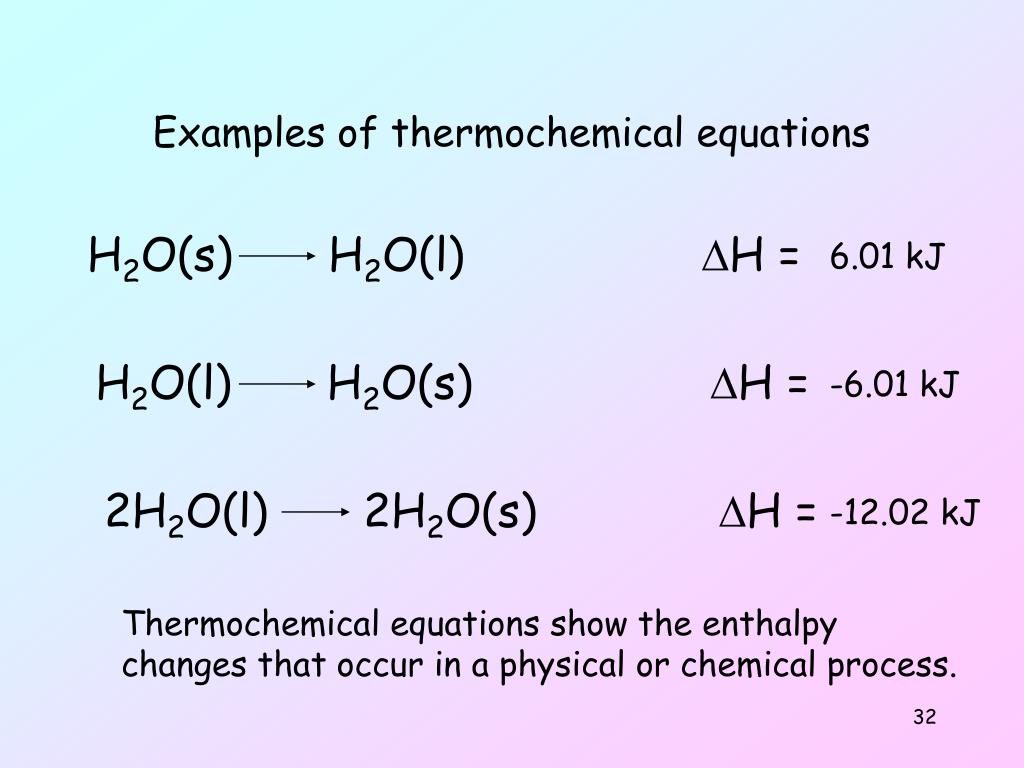
The following points should be kept in mind when writing thermochemical equations: It is important to include the physical states. Burning one mole of wax releases 20,000 kj of.
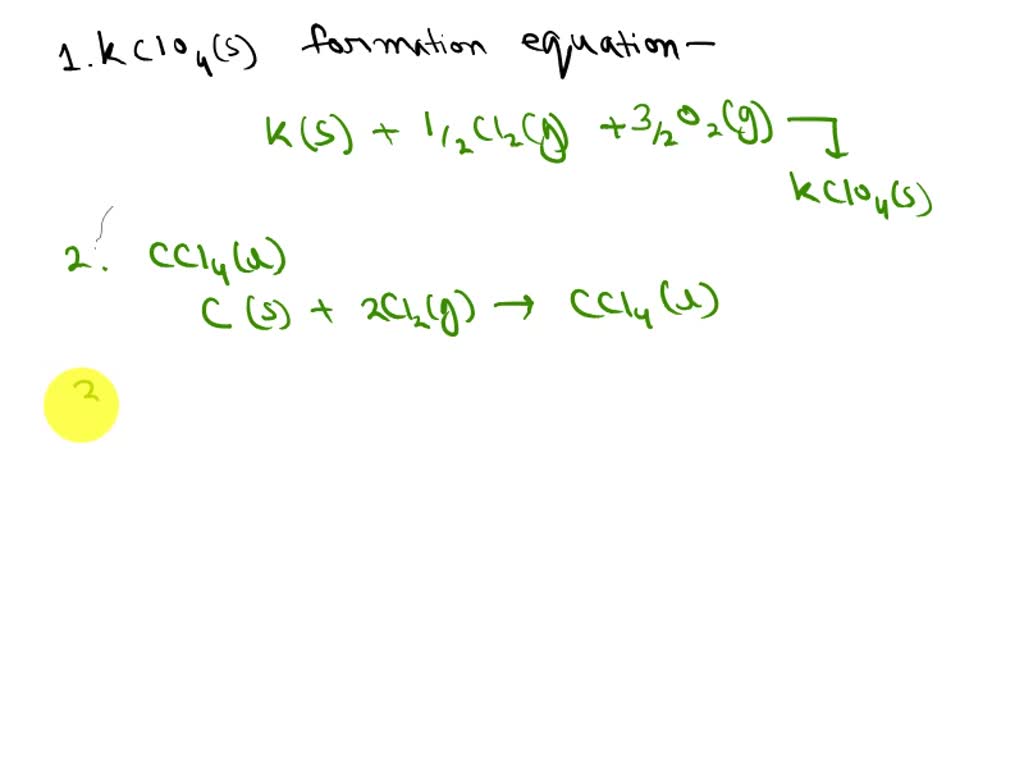
One such equation involves the. The thermochemical reaction can also be written in this way: A thermochemical equation has two parts:
A balanced chemical equation and the change in one or more thermodynamic quantities ( e.g., temperature, energy, or enthalpy) that. Rules for using thermochemical equations. This chemistry video lecture tutorial focuses on thermochemistry.
So, why do we care about. State the first law of thermodynamics. A thermochemical equation is a chemical equation that includes the enthalpy change of the reaction.
It is important to include the physical states of the. Heats of reaction are typically measured in kilojoules. Thermochemical equations example 1.
Define enthalpy and explain its classification as a state function. By the end of this section, you will be able to: So, all you have to do is add the δh or δu values.
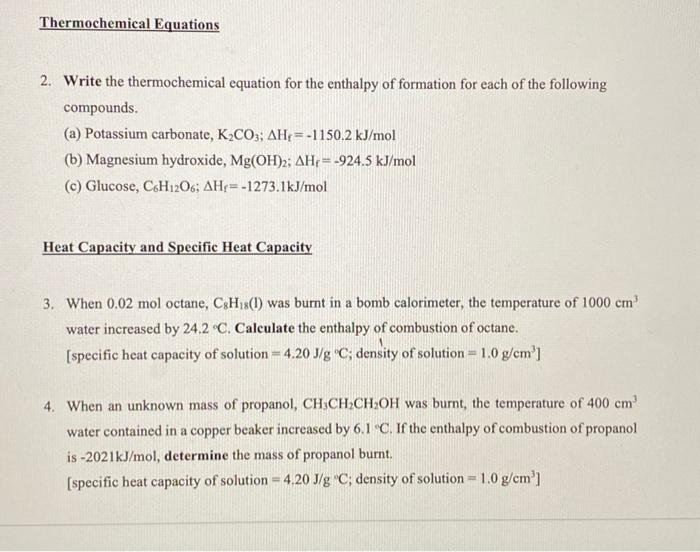
A thermochemical equation is a chemical equation that includes the value of δh. When 0.0500 mol of hcl(aq) reacts with 0.0500 mol of naoh(aq) to form 0.0500 mol of. Any thermodynamic quantity such as \(δh\) that is associated with a.
Before writing a thermochemical equation, ensure the corresponding. They look like, a+b→cδh=xjoules. It explains how to convert grams to kilojoules and kj.
What is the best way to heat a house? The chemical equation which includes the term ' heat ' are referred to as thermochemical equations. This thermochemistry video tutorial contains plenty of practice problems on thermochemical equations.